"The Matrikon OPC Server is doing well and has provided everything we need. The support we received from Matrikon was always prompt and very helpful."
René van Opstal
Project Manager, AllMons
AllMons, a leading process and automation consultant company, based in the Netherlands, was retained by Diosynth, a world leading bio-pharmaceutical company, to integrate process control systems within a new facility. The new infrastructure controlled and monitored plant processes and building management systems. In addition, the control systems were tied into a database for logging and reporting production data to ensure compliance with the FDA’s 21 CFR Part 11 regulations.
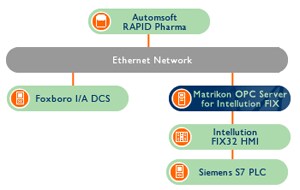 The plant is controlled by a Foxboro I/A DCS and Siemens S7 PLC, which use Intellution’s FIX32 HMI for visualization. A connectivity solution was needed to connect the HMI to the Automsoft RAPID Pharma database, an application which would log data from the control systems and generate reports.
The plant is controlled by a Foxboro I/A DCS and Siemens S7 PLC, which use Intellution’s FIX32 HMI for visualization. A connectivity solution was needed to connect the HMI to the Automsoft RAPID Pharma database, an application which would log data from the control systems and generate reports.
AllMons consultant René van Opstal investigated his options and chose Matrikon OPC to integrate the solution. Matrikon’s OPC Server for Intellution FIX was implemented to connect the FIX32 HMI to the RAPID Pharma database – an application that already supported OPC. In addition to providing OPC products that guarantee robust, reliable connections, Matrikon has been certified as a provider of 21 CFR Part 11-compliant technology and services.
"This was my first experience with Matrikon and it was good," van Opstal says. "The Matrikon OPC Server is doing well and has provided everything we need. The support we received from Matrikon was always prompt and very helpful. We plan to integ